Eye product recall: FDA expands warning over contaminated eye drops to include Delsam Pharma's Artificial Eye Ointment, amid bacterial outbreak - CBS News
The FDA says Delsam Pharma's Artificial Eye Ointment may be contaminated with bacteria, following outbreak of a drug-resistant strain.
The FDA says Delsam Pharma's Artificial Eye Ointment may be contaminated with bacteria, following outbreak of a drug-resistant strain.

Eye product recall: FDA expands warning over contaminated eye

Eye drops recall due to bacteria outbreak includes Texas
:max_bytes(150000):strip_icc()/salad-GettyImages-544116020-0078a96236dc43fca5287431390a2a57.jpg)
Eye Ointment Recall: Walmart, CVS Products May Pose Infection Risk

Eye product recall: FDA expands warning over contaminated eye

FDA issues warning for 26 eyedrops due to risk of infection

Southeastern Adds Franklinton's Smith to Coaching Staff

Eye product recall: FDA expands warning over contaminated eye

EzriCare & Delsam Artificial Tears Recall: FDA Eye Drop Recall

CBS Evening News With Norah O'Donnell : KPIX : February 22, 2023 6
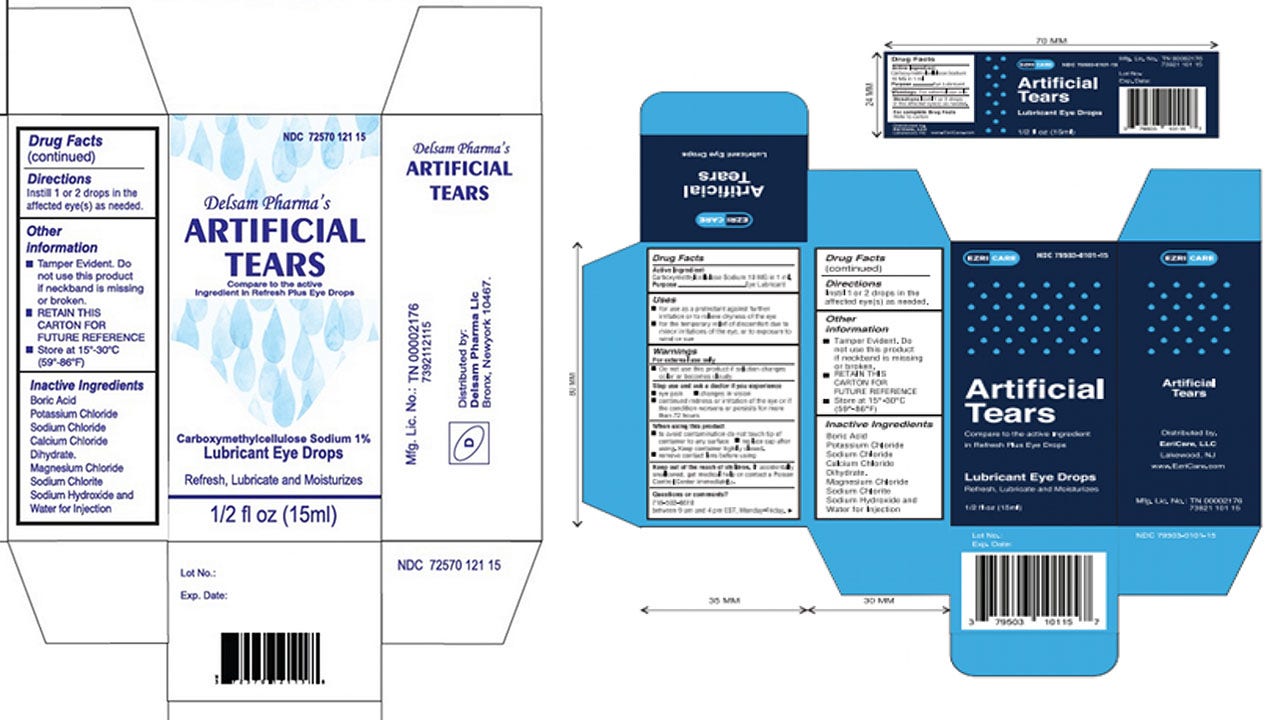
Eye drop manufacturer issues recall after US drug-resistant

FDA issues warning for 26 eyedrops due to risk of infection

EzriCare, Delsam Pharma eye drops recall in California
FDA issues warning for 26 eyedrops due to risk of infection

FDA expands recall of potentially contaminated eye products amid
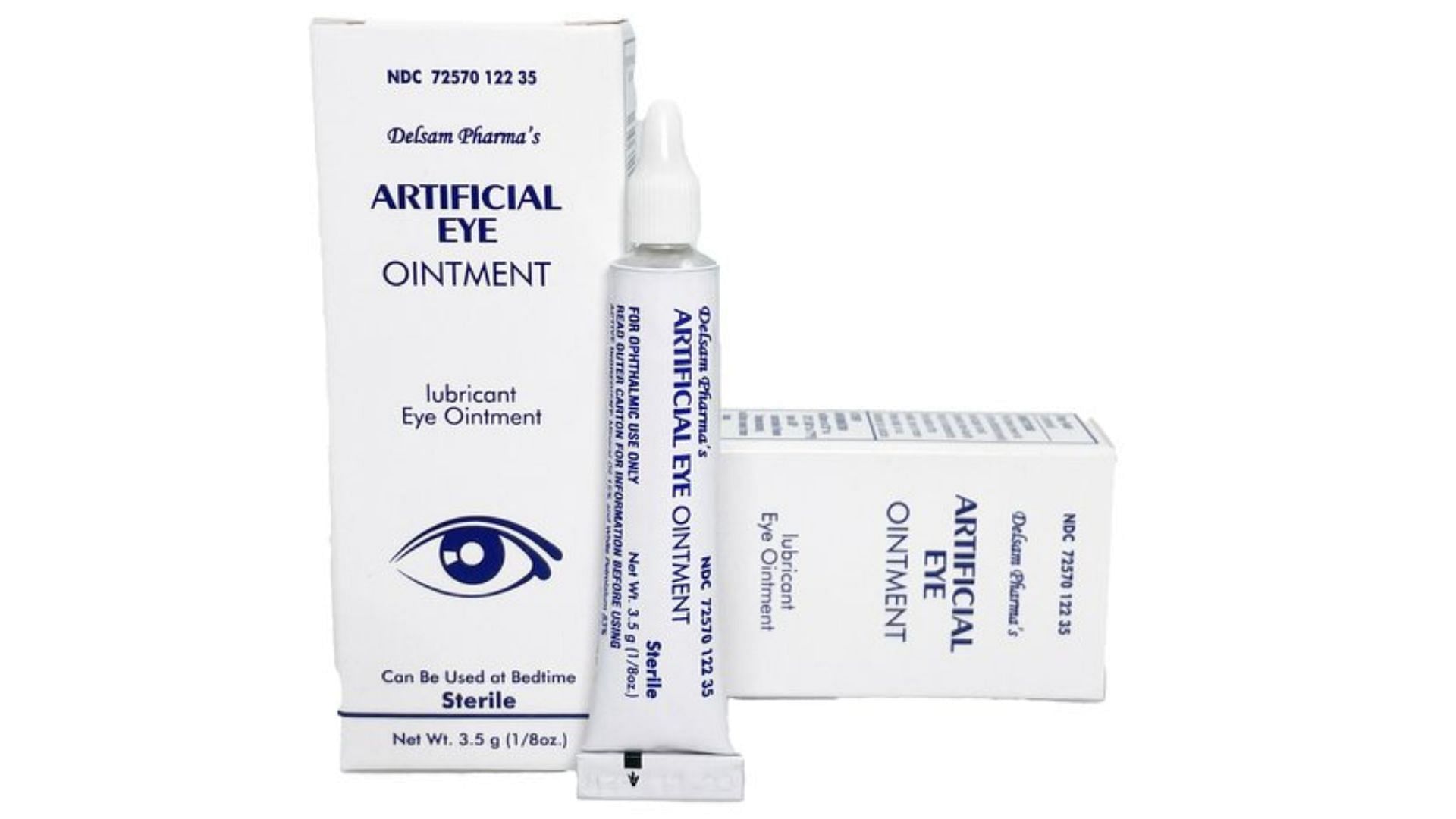
What eye drops are contaminated? FDA expands warning recall list